Business Process Mapping - Streamlining Management of Data/Records for a Clinical Trial

Business Process Mapping - Streamlining Management of Data/Records for a Clinical TrialПодробнее

Streamlining Clinical Trial AnalyticsПодробнее

The 5Vs of Data Management in Clinical TrialsПодробнее

Data Management & Case Report in Clinical Trials: Protocol and Data Collection Part 1Подробнее

IPPCR 2016: Data Management & Case Report Form Development in Clinical TrialsПодробнее

Clinical Trial Management System (CTMS) Data MigrationПодробнее

Process MappingПодробнее
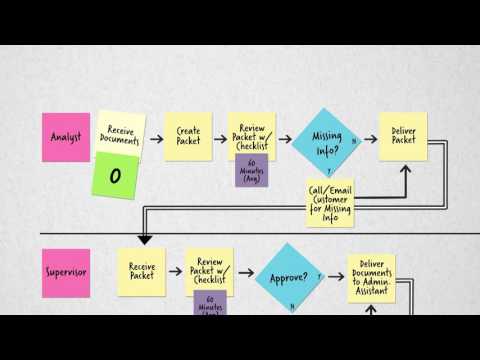
How Medtech Companies are Streamlining Clinical Trial Operations with Risk-Based StrategiesПодробнее

Widen the Clinical Trial Recruitment Funnel & Streamline Engagement & EnrollmentПодробнее

Sharing Clinical Trial Management System (CTMS) Data Between Sponsors and CROsПодробнее

The Essential Role of Data Management in Clinical TrialsПодробнее

Business Process Mapping 101 (Step By Step Guide)Подробнее
Why You Need a Clinical Trial Management System (CTMS)Подробнее

Quality and Control of Clinical Trial Data (6of11) GCP Data Integrity WorkshopПодробнее

Adverse Event in Clinical TrialsПодробнее

Value Stream Map - What is it? How do we use it?Подробнее

Archived Presentation: Refining the Clinical Trials Units for NIAID NetworksПодробнее

TCS BPS’ clinical data management meets patient centricity demandsПодробнее

IPPCR: Quality Management in Clinical ResearchПодробнее
