SCH3U1 - 8.6 Concentrations (Concentration vs Solubility calculations)

Solubility calculationsПодробнее

Solubility vs Concentration - Basic Introduction, Saturated Unsaturated and Supersaturated SolutionsПодробнее

Ksp - Molar Solubility, Ice Tables, & Common Ion EffectПодробнее
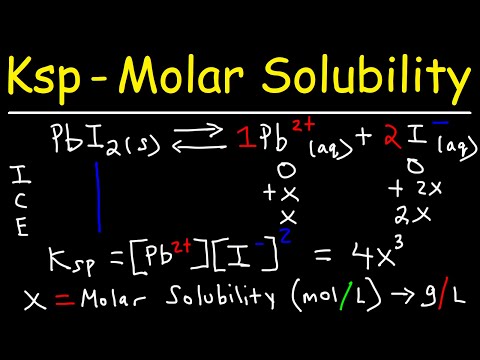
Chem 12 - U3b - Calculating Solubility and Ion ConcentrationsПодробнее

Worked example: Calculating solubility from Kₛₚ | Equilibrium | AP Chemistry | Khan AcademyПодробнее

37: Calculating solubility, molar solubility, and KspПодробнее

Solubility Product Constant (Ksp)Подробнее
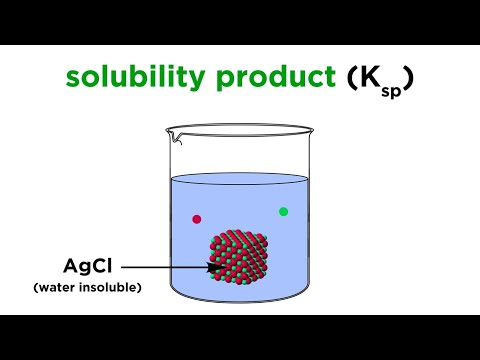
⚗️ Calculating Molar Solubility in the Presence of a Common IonПодробнее

Calculating Ks from solubility.Подробнее

Solubility CalculationsПодробнее

How to Calculate Solubility By the Systematic Method in ChemistryПодробнее

Calculate Moles of Solute from Molarity #science #chemistry #education #shorts #shortПодробнее

⚗️ Calculating Molar Solubility from Kₛₚ (Question 2)Подробнее

Calculate molar solubilityПодробнее

⚗️ Calculating Molar Solubility from Kₛₚ (Question 1)Подробнее

MCAT ACE Chemistry 15 - Solutions and Solubility: Concentrations, Dilutions, and Ksp ExplainedПодробнее

How to Calculate Molar Solubility from KspПодробнее

Problem 6.28Calculate the molar solubility of Ni(OH)2 in 0.10 M NaOH. The ionic product of Ni(OH)2Подробнее
