15.11d | Calculate the solubility product for La2(MoO4)3, 0.00179 g/100 mL

15.11c | Calculate the solubility product for NH4MgAsO4·6H2O, 0.038 g/100 mLПодробнее

15.10c | Calculate the solubility product for Gd2(SO4)3, 3.98 g/100 mLПодробнее

15.10a | Calculate the solubility product for BaSiF6, 0.026 g/100 mL (contains SiF6 2− ions)Подробнее

15.11a | Calculate the solubility product for BaSeO4, 0.0118 g/100 mLПодробнее

15.10b | Calculate the solubility product for Ce(IO3)4, 1.5 × 10^–2 g/100 mLПодробнее

Worked example: Calculating solubility from Kₛₚ | Equilibrium | AP Chemistry | Khan AcademyПодробнее

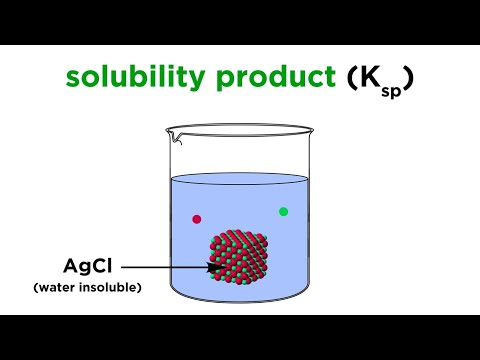
Solubility Product Constant (Ksp)Подробнее
15.10d | Calculate the solubility product for (NH4)2PtBr6, 0.59 g/100 mL (contains PtBr6 2− ions)Подробнее

15.11b | Calculate the solubility product for Ba(BrO3)2·H2O, 0.30 g/100 mLПодробнее

17.5.11 Solubility of Al(OH)3 in 15 M NH3 Part IIПодробнее

⚗️ Calculating Molar Solubility from Kₛₚ (Question 2)Подробнее

Formative: 200mL of 0.05 M Na2S and 100 mL of 0.002M AgNO3. Find the mass of any precipitate formed.Подробнее

A sample of AgCl was treated with 5.00ML of 1.5 M Na2CO3 solution || Solubility Product| EquilibriumПодробнее

Calculate the pH of the following buffer solutions. (a) 0.050 M in sodium formate, NaCHO2, and 0.10…Подробнее

5 15 2Подробнее

Problem 1.71 For a certain liquid µ = 7.1 × 10-5 lbs/ft^2 at 40 °F and µ =1.9 x 10-5 lbs/ft^2 at 15…Подробнее

The mass in grams of 2.6times 10^22 chlorine atoms is: A 3.2 B 0.76 C ) 11 D ) 4.4 E ) 1.5Подробнее

14.04 The Solubility Product ConstantПодробнее
